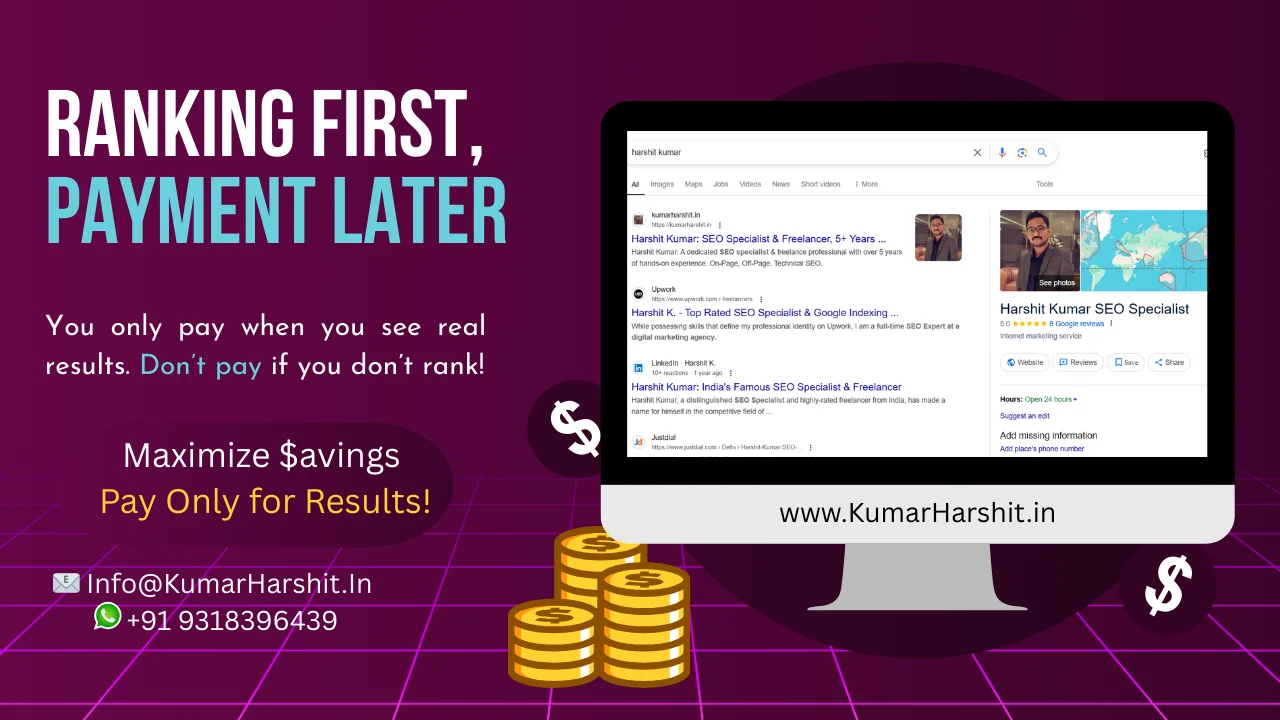
Textile products can be tested for the virucidal activity against specified viruses using ISO 18184. All textiles, knitted fabrics, and other related products must adhere to this standard. Results from one virus can’t be applied to other viruses because of their varying sensitivity levels. As living standards rise around the world, more people are looking for health-protective products, which has led to an increase in demand for ISO 18184.
Because they are relatively new products, it is necessary to create test methods to determine whether or not they are effective. As their name implies, antiviral textile products are those whose surface area they can prevent from being infected by infectious viruses.
The plate and TCID50 methods are included in the ISO 18184 standard, which specifies two measurement methods. There are as many as two ways that labs use depending on their level of experience.
ISO Standards and Specifications for the Testing and Evaluation of Textile Antiviral Activity.
Items that are porous and textiles have virucidal antimicrobial functions. This is calculated via the antiviral textile test that is named ISO 18184. How well these materials are able to diminish the effects of viruses are tested with ISO 18184 standard test. Flu and coronavirus strains are two of the most commonly tested viruses.
Most Common Antimicrobial Additives
Whenever there is an antiviral function, it is indicated because of the chemicals which, in some way, can make integral changes to the virus’s chemical structure. Receptor-ligand binding is a drug strategy that uses a ligand that binds to a specific receptor on the virus to block that receptor’s mode of action.
Chemically targeting a specific virus aspect is a more common method for treating products. Essential oils, quaternary-silane molecules, quaternary ammonium compounds, and quaternary-silane molecules are standard antimicrobials used in textiles, all of which are chemically related to surfactants or detergents.
Oxidation is also a key process. It can be executed on the surface of the material after which the virus becomes dormant. Chemicals like hypochlorite or bleach belong to specific antiviral and antimicrobial compounds that employ this strategy. Due to their durability, these are used less frequently, but they are still an effective cleaning method.
When a product’s antimicrobial performance against viruses is tested in an environmental setting, many customers will ask for durability testing as part of the ISO 18184 method. Utilizing Situ Biosciences’ product test laboratory to conduct durability and antimicrobial testing can help with product development and performance testing while also cutting down on the time and costs associated with creating and distributing high-quality products.
Foreword
Government and non-governmental agencies from around the world also play a role. We work closely together with the IEC on all aspects of the standardization of electromechanical devices.Part 1 of the ISO/IEC Directives outlines the procedures used to create and maintain this document. It’s essential to take note of the various approval criteria required for several types of ISO documents.
· A trade name in this document does not imply an endorsement of the product or service.
· Technical Committee ISO/TC 38, Textile, drafted this document.
The following are the most significant changes from the previous edition:
· There has been a change to Clause 1;
· There is no longer a section in 10.6 entitled Verification of cytotoxic effect.
· There has been an update to 11.1, Preparation of specimen.
· It has been updated in 14.3.2, Calculation of antiviral activity value.
· These organizations are all listed on the ISO website at www.iso.org/members.html.
Health Care:
Consumers have recently shown a trend toward seeking healthcare or health-protective products, as living standards around the world have improved. There has also been increased public concern about disease outbreaks, as evidenced by the overcrowding in subway cars, hospitals, and other care facilities frequented by commuters daily.
With the help of recently improved textile processing technology, health-protective and hygiene-related products have made significant strides in the market.
Individual manufacturers have developed testing methods because those products are relatively new and include the technical aspects of textile technology. As a result, there is no standardized way to test these high-functioning products, making it difficult for both consumers and manufacturers to get a clear picture of what they are.
An antiviral product incorporating textile and biotechnology design elements is one of these items. Some textiles can reduce the number of virus particles that come into contact with their surface. Because of the data presented in this document, everyone interested in antiviral textile products can now better understand the products’ actual performance.
Study of The Antiviral Activities
The antiviral activity of textile products can be determined using the methods outlined in this document. Fabrics, yarns, braids, and other textile components are all included in this category.
Necessary Definitions To Understand
There are particular definitions and some explanations that are necessary to understand before moving ahead in the process. The terms are most commonly used during the process.
1. The Common Virus
Viruses work in a different manner. Their method of reproduction happens at a cellular level. The genetic material of the virus combines with it’s opposing host to duplicate.
2. Infected organisms
Using this test is being able to find out about cells that are both susceptible and permissive.
3. Ability to fight viruses
Substance (chemical or otherwise) causes a change in the virion structure that prevents it from replicating. One can reduce viral activity by altering the virus’s shape or causing structural damage to its surface protein. Virus infectivity may decrease, but this isn’t always the case by a shift in antigenic response or a change in a virus’s constituent element.
4. Anthracyclines
Antiviral chemicals that use chemical adsorption change the virus’s surface protein by altering it. They destroy or limit the virus’s morphology by removing hydrogen atoms from the virus protein through the radical reaction, generating ohmic (OH) radicals.
5. Inhibitor cloth
This test is used to check for virus stability on a textile fabric. The cloth used in this case is a control. In other words, a common fabric on which no tests have been performed. Using the control cloth as the base, tests are performed on it.
6. A measure of quality assurance
Confirm that a specimen does not harm the host cell by performing this test. This test is identical to the actual test, except that the virus has been omitted. To be added as a second note to this entry: Often referred to as a specimen control test.
7. A cytotoxic effect on cells
Alterations in cell morphology and cell destruction or a decrease in the sensitivity of cells to viral multiplication caused by a product. The virus’s ability to multiply causes changes in the host cells’ morphology or even their death. Using this test it is easy to find the effect of cytotoxic cells.
8. The examination of plaque
Virus infectivity titer can be assayed using a series of dilutions from PFU which is followed by this test.
9. TCID50
Wash-out virus suspension or dilution of virus suspension induces CPE in 50 percent of cell culture units with 50 percent infectious dose.
10. The TCID50 method
Infectivity titer (3.8) of a virus is determined using the serial dilution assay from TCID50